
Back التحليل الكهربائي ذو درجة الحرارة العالية Arabic Hochtemperaturelektrolyse German Electrólisis de alta temperatura Spanish Korkean lämpötilan elektrolyysi Finnish Électrolyse à haute température French Elettrolisi ad alta temperatura Italian Hogetemperatuurelektrolyse Dutch Высокотемпературный электролиз Russian

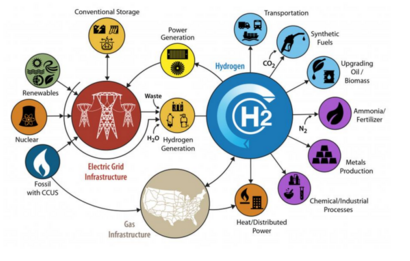
High-temperature electrolysis (also HTE or steam electrolysis, or HTSE) is a technology for producing hydrogen from water at high temperatures or other products, such as iron or carbon nanomaterials, as higher energy lowers needed electricity to split molecules and opens up new, potentially better electrolytes like molten salts or hydroxides.[1][2][3][4] Unlike electrolysis at room temperature, HTE operates at elevated temperature ranges depending on the thermal capacity of the material.[5] Because of the detrimental effects of burning fossil fuels[6] on humans and the environment, HTE has become a necessary alternative and efficient method by which hydrogen can be prepared on a large scale and used as fuel. The vision of HTE is to move towards decarbonization[7][8] in all economic sectors. The material requirements for this process are: the heat source, the electrodes, the electrolyte, the electrolyzer membrane, and the source of electricity.
- ^ Hauch, A.; Ebbesen, S. D.; Jensen, S. H.; Mogensen, M. (2008). "Highly Efficient high temperature electrolysis". J. Mater. Chem. 18 (20): 2331–2340. doi:10.1039/b718822f.
- ^ Licht, Stuart; Wu, Hongjun (2011-12-22). "STEP Iron, a Chemistry of Iron Formation without CO 2 Emission: Molten Carbonate Solubility and Electrochemistry of Iron Ore Impurities". The Journal of Physical Chemistry C. 115 (50): 25138–25147. doi:10.1021/jp2078715. ISSN 1932-7447.
- ^ Licht, Stuart; Cui, Baochen; Wang, Baohui (2013-09-01). "STEP carbon capture – The barium advantage". Journal of CO2 Utilization. 2: 58–63. Bibcode:2013JCOU....2...58L. doi:10.1016/j.jcou.2013.03.006. ISSN 2212-9820.
- ^ Ren, Jiawen; Yu, Ao; Peng, Ping; Lefler, Matthew; Li, Fang-Fang; Licht, Stuart (2019-11-19). "Recent Advances in Solar Thermal Electrochemical Process (STEP) for Carbon Neutral Products and High Value Nanocarbons". Accounts of Chemical Research. 52 (11): 3177–3187. doi:10.1021/acs.accounts.9b00405. ISSN 0001-4842. PMID 31697061.
- ^ Valderrama, César (2016), "High-Temperature Electrolysis", in Drioli, Enrico; Giorno, Lidietta (eds.), Encyclopedia of Membranes, Berlin, Heidelberg: Springer, pp. 937–939, doi:10.1007/978-3-662-44324-8_2122, ISBN 978-3-662-44324-8, retrieved 2024-04-14
- ^ "Fact Sheet | Climate, Environmental, and Health Impacts of Fossil Fuels (2021) | White Papers". www.eesi.org. Retrieved 2024-04-14.
- ^ Shiva Kumar, S.; Lim, Hankwon (November 2022). "An overview of water electrolysis technologies for green hydrogen production". Energy Reports. 8: 13793–13813. Bibcode:2022EnRep...813793S. doi:10.1016/j.egyr.2022.10.127. ISSN 2352-4847.
- ^ Zainal, Bidattul Syirat; Ker, Pin Jern; Mohamed, Hassan; Ong, Hwai Chyuan; Fattah, I.M.R.; Rahman, S.M. Ashrafur; Nghiem, Long D.; Mahlia, T M Indra (January 2024). "Recent advancement and assessment of green hydrogen production technologies". Renewable and Sustainable Energy Reviews. 189: 113941. Bibcode:2024RSERv.18913941Z. doi:10.1016/j.rser.2023.113941. ISSN 1364-0321.